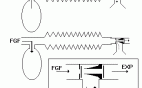
ANAESTHETIC BREATHING SYSTEMS
DR M Ravi Shankar
Professor and chair of Anesthesia
JIPMER, Pondicheryy, India
Introduction
Since the introduction of diethyl ether as an anaesthetic in 1846, the speciality of anaesthesia has come a long way. In the initial phase, the attention was mainly diverted to administering a single agent and apparatus were developed to suit the purpose. The reintroduction of nitrous oxide in 1868, and facility to store it in cylinders, created an interest in administering a combination of agents to anaesthetise patients. Any resemblance to a breathing system was developed by Barth (1907) using his valve with a nitrous oxide cylinder, a reservoir bag and Clover’s inhaler. Different positions of the lever in the valve allowed a range from complete rebreathing to completely breathing from the atmosphere. The development of Boyle’s machine (1917) and mastering of endotracheal intubation with a soft red rubber single lumen tube by Magill and Rowbothom were the forerunners for development of a simple anaesthetic delivery system by Magill, popularly known as the “Magill’s circuit”. Introduction of cyclopropane in 1929 and cuffed endotracheal tube in 1931; prompted Waters to develop the “to and fro” canister and use it for closed system anaesthesia with cyclopropane. In 1936, Brian Sword introduced the circle system. The Ayre’s T-piece was introduced in 1937, EMO inhaler in 1941 and Minnitt’s ‘gas and air’ apparatus with demand valve in 1949.
With the introduction of many breathing systems attempts were made to classify them in the 50’s and 60’s, but lack of a proper definition, lead to more confusion than clarity.
DEFINITION:
A breathing system is defined as an assembly of components which connects the patient’s airway to the anaesthetic machine creating an artificial atmosphere, from and into which the patient breathes.
It primarily consists of
a) A fresh gas entry port/delivery tube through which the gases are delivered from the machine to the systems;
b) A port to connect it to the patient’s airway;
c) A reservoir for gas, in the form of a bag or a corrugated tube to meet the peak inspiratory flow requirements;
d) An expiratory port/valve through which the expired gas is vented to the atmosphere;
e) A carbon dioxide absorber if total rebreathing is to be allowed and
f) Corrugated tubes for connecting these components.
Flow directing valves may or may not be used.
REQUIREMENTS OF A BREATHING SYSTEM:
The components when assembled should satisfy certain requirements, some essential and others desirable.
Essential:
The breathing system must
a) deliver the gases from the machine to the alveoli in the same concentration as set and in the shortest possible time;
b) effectively eliminate carbon-dioxide;
c) have minimal apparatus dead space; and
d) have low resistance.
Desirable:
The desirable requirements are
a) economy of fresh gas;
b) conservation of heat;
c) adequate humidification of inspired gas;
d) Light weight;
e) convenience during use;
f) efficiency during spontaneous as well as controlled ventilation (Efficiency is determined in terms of co2 elimination and fresh gas utilization);
g) adaptability for adults, children and mechanical ventilators;
h) provision to reduce theatre pollution.
CLASSIFICATION OF BREATHING SYSTEMS:
One will realize the reason for the failure of the attempts at classification in the 50’s to 60’s, if this definition and requirements are taken into account. There are numerous classifications of breathing systems according to the whims and fancy of the person classifying. Many of them are irrelevant as they do not define a breathing system. Different authors classified the same system under different headings, adding to confusion1. McMohan in 1951 classified them as open, semiclosed and closed taking the level of rebreathing into account. It as follows:
| Open | no rebreathing |
| Semiclosed | partial rebreathing |
| Closed | total rebreathing |
Dripps et al have classified them as Insufflation, Open, Semiopen, Semiclosed and Closed taking into account the presence or absence of Reservoir, Rebreathing, co2 absorption and Directional valves1. The ambiguity of the terminology used as open, semi open, semi closed and closed allowed inclusion of apparatus that are not breathing systems at all into the classification.
To overcome this problem Conway2 suggested that a functional classification be used and classified according to the method used for co2 elimination as:
1. Breathing systems with co2 absorber and
2. Breathing systems without co2 absorber.
Miller.D.M.3 in 1988 widened the scope of this classification so as to include the enclosed afferent reservoir system.
A new breathing system called ‘The Maxima’4 has been designed by Miller in 1995 and to include it in the classification5, the enclosed afferent reservoir systems have been grouped under ‘displacement afferent reservoir’ systems.
| BREATHING SYSTEMS WITHOUT co2 ABSORPTION. |
I BREATHING SYSTEMS WITH co2 ABSORPTION.
|
| Unidirectional flow:
a) Non rebreathing systems. |
Unidirectional flow
Circle system with absorber. |
Bi-directional flow:a) Afferent reservoir systems.
B) Enclosed afferent reservoir systems
c) Efferent reservoir systems
d) Combined systems
|
Bi-directional flow
|
This classification also has a personal bias as the Humphrey ADE system is not included in the classification, even though he preferred to compare his system with that of Humphrey’s6. The classification suggested in table.1. is a partial modification of Miller’s3 classification.
BREATHING SYSTEMS WITHOUT co2 ABSORPTION
Unidirectional Flow
a) Nonrebreathing systems.
They use non rebreathing valves and there is no mixing of fresh gas and the expired gas.
Functional analysis: When the patient takes a breath, or if the reservoir bag is squeezed, the inspiratory unidirectional valve opens and the gases flow into the patient’s lungs(Fig.1). The expiratory unidirectional valve closes the expiratory port during spontaneous breathing. The inspiratory unidirectional valve itself closes the expiratory port during controlled ventilation. At the start of expiration, the inspiratory unidirectional valve returns back to position and expiration takes place through the expiratory port, opening the expiratory valve.
The fresh gas flow (FGF) should be equal to the minute ventilation (MV) of the patient. These systems satisfy all four essential requirements, but are not very popular because of the following reasons:
1) Fresh gas flow has to be constantly adjusted and is not economical.
2) There is no humidification of inspired gas.
3) There is no conservation of heat.
4) They are not convenient as the bulk of the valve has to be positioned near the patient.
5) The valves can malfunction due to condensation of moisture and lead to complications.
B) Circle Systems:
These systems are designed with a co2 absorber as an essential component of the system. To use it without absorber is uneconomical as it needs a FGF more than the alveolar ventilation.
The effect of arrangements of various components in the effective elimination of co2 and fresh gas economy when used with high flows were analysed by Egar and Ethans7. Detailed discussion on this is beyond the purview of this review. However, some aspects of this is discussed under the section, Circle system with absorber.
Bi-Directional Flow:
Systems with bi-directional flow are extensively used. These systems depend on the FGF for effective elimination of co2. Understanding these systems is most important as their functioning can be manipulated by changing parameters like Fresh gas flow, alveolar ventilation, apparatus dead space, etc. We will analyze these in detail.
Fresh Gas Supply; Fresh gas flow (FGF) forms one of the essential requirements of a breathing system. If there is no FGF into the system, the patient will get suffocated. If the FGF is low, most systems do not eliminate carbon-dioxide effectively, and if there is an excess flow there is wastage of gas. So, it becomes imperative to specify optimum FGF for a breathing system for efficient functioning.
If the system has to deliver a set concentration in the shortest possible time to the alveoli, the FGF should be delivered as near the patient’s airway as possible.
Elimination Of Carbon-Dioxide: The following may be taken as an example for better understanding of co2 elimination by the bi-directional flow systems. Normal production of carbon-dioxide in a 70 kg adult is 200 ml per minute and it is eliminated through the lungs. Normal end-tidal concentration of carbon-dioxide is 5%. Hence, for eliminating 200 ml of carbon-dioxide as a 5% gas mixture, the alveolar ventilation has to be:
200 x 100 = 4,000 ml
5
This 4000 ml or 4 litres is the normal alveolar ventilation. Any breathing system connected to an adult’s airway should provide a minimum of 4 litres per minute of carbon-dioxide free gas to the alveoli for eliminating carbon-dioxide. If the alveolar ventilation becomes less than 4 litres per minute, it would lead to hypercarbia. If the alveoli are ventilated with 5 litres/minute of a gas containing 1% carbon-dioxide, or 8 litres/minute of a gas containing 2.5% carbon-dioxide, it could still eliminate 200 ml of carbon-dioxide per minute from the alveoli. It may be construed as 4 litres of co2 free gas and 1 litre of gas with 5% co2 in the first instant and as 4 litres of co2 free gas and 4 litres of gas with 5% co2 in the second instant. In effect, 4 litres of alveolar ventilation with co2 free gas is provided in both cases.
Apparatus Dead Space: It is the volume of the breathing system from the patient-end to the point up to which, to and fro movement of expired gas takes place.
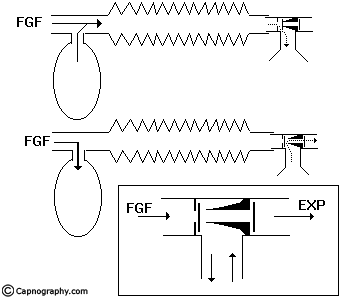
In an afferent reservoir system with adequate FGF, the apparatus dead space extends up to the expiratory valve positioned near the patient (fig.2).
If the FG enters the system near the patient-end as in an efferent reservoir system, the dead space extends upto the point of FG entry. In systems where inspiratory and expiratory limbs are separate, it extends upto the point of bifurcation. The dynamic dead space will depend on the FGF and the alveolar ventilation. The dead space is minimal with optimal FGF. If the FGF is reduced below the optimal level, the dead space increases and the whole system will act as dead space if there is no FGF. Increasing the FGF above the optimum level will only lead to wastage of FG.
Sub-Classification Of Bi-Directional Flow Systems:
Mapleson8 did a theoretical analysis of the fresh gas requirements of the semiclosed systems available at that time. It is only proper to refer to it as Mapleson systems as he gave a nomenclature as A, B, C, D and E for easy identification as per their construction. For better understanding of the functional analyses, they have been classified as:
| 1 | Afferent reservoir system (ARS). |
| 2 | Enclosed afferent reservoir systems (EARS). |
| 3 | Efferent reservoir systems (ERS). |
| 4 | Combined systems. |
The afferent limb is that part of the breathing system which delivers the fresh gas from the machine to the patient. If the reservoir is placed in this limb as in Mapleson A, B, C and Lack’s systems, they are called afferent reservoir systems (ARS).
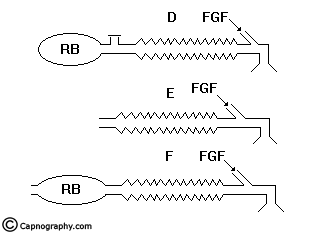
The efferent limb is that part of the breathing system which carries expired gas from the patient and vents it to the atmosphere through the expiratory valve/port. If the reservoir is placed in this limb as in Mapleson D, E, F and Bain systems, they are called efferent reservoir systems (ERS).
Enclosed afferent reservoir system has been described by Miller and Miller.
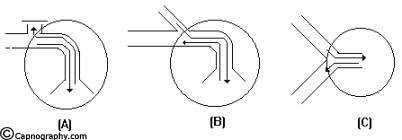
AFFERENT RESERVOIR (AR) SYSTEMS
The Mapleson A, B and C systems have the reservoir in the afferent limb, and do not have an efferent limb (Fig.3). Lack system has an afferent limb reservoir and an efferent limb through which the expired gas traverses before being vented into the atmosphere (Fig.4). This limb is coaxially placed inside the afferent limb.
These AR systems work efficiently during spontaneous breathing provided the expiratory valve is separated from the reservoir bag and FGF by at least one tidal volume of the patient and apparatus dead space is minimal. They do not function efficiently during controlled ventilation. If the FGF is close to the expiratory valve as in Mapleson B & C, the system is inefficient both during spontaneous and controlled ventilation. The efficiency is determined in terms of carbon-dioxide elimination and FGF utilization.
Mapleson8 has analysed these bi-directional flow systems using mathematical calculations. He made a few basic assumptions while analyzing breathing systems. These are
(1) Gases move enbloc. They maintain their identity as fresh gas, dead space gas and alveolar gas. There is no mixing of these gases.
(2) The reservoir bag continues to fill up, without offering any resistance till it is full.
(3) The expiratory valve opens as soon as the reservoir bag is full and the pressure inside the system goes above atmospheric pressure.
(4) The valve remains open throughout the expiratory phase without offering any resistance to gas flow and closes at the start of the next inspiration.
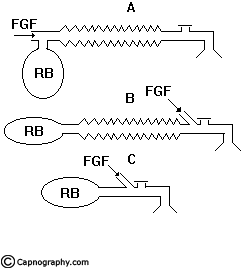
Mapleson ‘A’/Magill’s system:
Functional analysis:
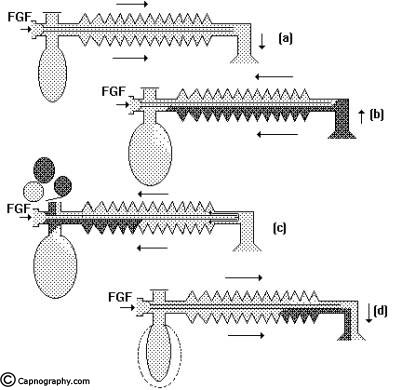
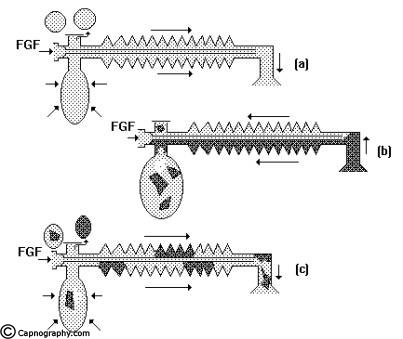
Spontaneous breathing: The system is filled with fresh gas before connecting to the patient. When the patient inspires, the fresh gas from the machine and the reservoir bag flows to the patient, and as a result the reservoir bag collapses (Fig.5a). During expiration, the FG continues to flow into the system and fill the reservoir bag. The expired gas, initial part of which is the dead space gas, pushes the FG from the corrugated tube into the reservoir bag and collects inside the corrugated tube (Fig.5b).
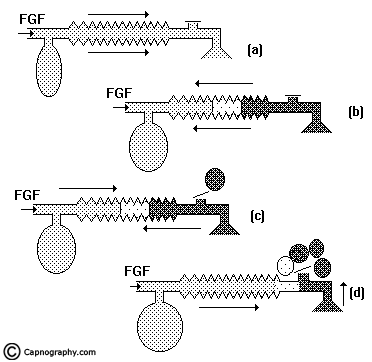
As soon as the reservoir bag is full, the expiratory valve opens and the alveolar gas is vented into the atmosphere (Fig.5c). During the expiratory pause, alveolar gas that had come into the corrugated tube is also pushed out through the valve, depending on the FGF. The system is filled with only fresh gas and dead space gas at the start of the next inspiration when FGF is equal to the alveolar ventilation (Fig.5d). The entire alveolar gas and dead space gas is vented through the valve and some FG also escapes, if the FGF is higher than the minute ventilation. Some amount of alveolar gas will remain in the system and lead to rebreathing with a FGF less than the alveolar ventilation. This has been confirmed theoretically and experimentally by many investigators8,9. The system functions at maximum efficiency, when the FGF equals the alveolar ventilation and the dead space gas (which has not taken part in gas exchange) is allowed to be rebreathed and utilized for alveolar ventilation.
Controlled ventilation: To facilitate IPPV the expiratory valve has to be partly closed. During inspiration, the patient gets ventilated with FG and part of the FG is vented through the valve (Fig.6a) after sufficient pressure has developed to open the valve. During expiration, the FG from the machine flows into the reservoir bag and all the expired gas (i.e., dead space gas and alveolar gas) flows back into the corrugated tube till the system is full (Fig.6b). During the next inspiration the alveolar gas is pushed back into the alveoli followed by the FG. When sufficient pressure is developed, part of the expired gas and part of the FG escape through the valve (Fig.6c). This leads to considerable rebreathing, as well as excessive waste of fresh gas. Hence these systems are inefficient for controlled ventilation.
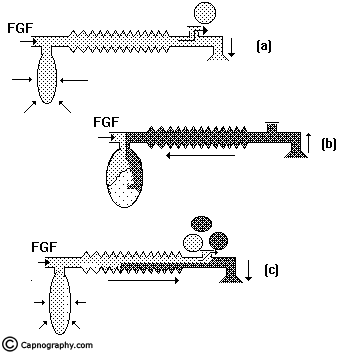
Lack’s system:
This system functions like a Mapleson A system both during spontaneous and controlled ventilation. The only difference is that the expired gas instead of getting vented through the valve near the patient, is carried by an efferent tube placed coaxially and vented through the valve placed near the machine end (Fig.4). This facilitates easy scavenging of expired gas.
Mapleson B & C systems:
In order to reduce the rebreathing of alveolar gas and to improve the utilization of FG during controlled ventilation, the FG entry was shifted near the patient(Fig.3). This allows a complete mixing of FG and expired gas. The end result is that these systems are neither efficient during spontaneous nor during controlled ventilation.
ENCLOSED AFFERENT RESERVOIR (EAR) SYSTEMS
This has been described by Miller & Miller10. The system consisted of a Mapleson A system enclosed within a non distensible structure (Fig.7a). It may also be constructed by enclosing the reservoir bag alone in a bottle and connecting the expiratory port to the bottle with a corrugated tube and a one way valve (Fig.7b). To the bottle is also attached a reservoir bag and a variable orifice for providing positive pressure ventilation.
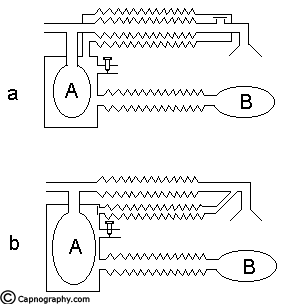
Functional analysis: During spontaneous ventilation, the gas is vented from the system in a manner which is identical to the Mapleson A system. In this mode the variable orifice is kept widely open to allow free communication to the atmosphere. In controlled ventilation the reservoir bag ‘B’ is squeezed intermittently and the variable orifice is partly closed to allow building up of pressure in the bottle. The pressure thus developed (1) closes the expiratory valve, (2) squeezes the enclosed afferent reservoir and the patient gets ventilated. The expiration takes place in a manner similar to that described during spontaneous ventilation when the pressure is released in reservoir ‘B’,. Hence this system should function efficiently during spontaneous and controlled ventilation with a FGF equivalent to alveolar ventilation. The fresh gas requirement and the utilization of this system has been investigated by a group of investigators from Manchester11-13 and a group from Wales14 under the guidance of Mapleson. They have reported varying figures for utilization as 82%, 93% and 74% respectively11,12,14. The reasons for this lesser percentage of utilization have been quoted as faulty methodology for calculation15, resistance offered by the reservoir bag and tubing and early opening of the unidirectional valve during expiration14 etc. Though the fresh gas requirement is higher than the alveolar ventilation in this system as shown by the above studies, it is still more efficient than the Bain system for controlled ventilation.
EFFERENT RESERVOIR (ER) SYSTEMS:
Bain system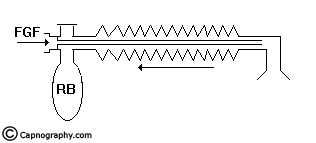
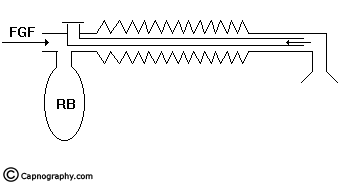
The Mapleson D, E, F and Bain systems have a 6 mm tube as the afferent limb that supplies the FG from the machine. The efferent limb is a wide-bore corrugated tube to which the reservoir bag is attached and the expiratory valve is positioned near the bag. In Mapleson E system, the corrugated tube itself acts as the reservoir (Fig.8). In Bain system, the afferent and efferent limbs are coaxially placed (Fig.9).
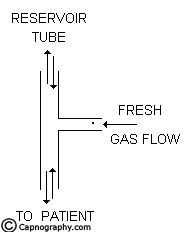
In an attempt to reduce FGF requirements, ER systems are constructed with reservoirs in the efferent limb. The functioning of all these systems are similar. These systems work efficiently and economically for controlled ventilation as long as the FG entry and the expiratory valve are separated by a volume equivalent to atleast one tidal volume of the patient. They are not economical during spontaneous breathing.
Spontaneous respiration: The breathing system should be filled with FG before connecting to the patient. When the patient takes an inspiration, the FG from the machine, the reservoir bag and the corrugated tube flow to the patient (Fig.11a). During expiration, there is a continuous FGF into the system at the patient end. The expired gas gets continuously mixed with the FG as it flows back into the corrugated tube and the reservoir bag (Fig.11b). Once the system is full the excess gas is vented to the atmosphere through the valve situated at the end of the corrugated tube near the reservoir bag. During the expiratory pause the FG continues to flow and fill the proximal portion of the corrugated tube while the mixed gas is vented through the valve (Fig.11c). During the next inspiration, the patient breaths FG as well as the mixed gas from the corrugated tube (Fig.11d). Many factors influence the composition of the inspired mixture. They are FGF, respiratory rate, expiratory pause, tidal volume and co2 production in the body. Factors other than FGF cannot be manipulated in a spontaneously breathing patient. It has been mathematically calculated and clinically proved8,16 that the FGF should be atleast 1.5 to 2 times the patient’s minute ventilation in order to minimise rebreathing to acceptable levels.
Factors that influence the composition of gas mixture in the corrugated tube with which the patient gets ventilated are the same as for spontaneous respiration namely FGF, respiratory rate, tidal volume and pattern of ventilation. The only difference is that these parameters can be totally controlled by the anaesthesiologist and do not depend on the patient. Using a low respiratory rate with a long expiratory pause and a high tidal volume, most of the FG could be utilized for alveolar ventilation without wastage.
Analyzing the performance of these systems during controlled ventilation, two relationships have become evident. 1) When FGF is very high the Paco2 becomes ventilation dependent (as during spontaneous respiration). 2) When the minute volume exceeds the FGF substantially, the Paco2 is dependent on the FGF17. Combining these influences a graph can be constructed as shown in Fig.13. An infinite number of combinations of FGF and minute ventilation can be chosen to achieve a desired Paco2. One can use a high FGF and a normal minute volume of 70 ml/kg to achieve a normal Paco2 of 40 mm Hg. This is uneconomical and leads to low humidity and heat loss. Alternately, a FGF equivalent to the predicted minute volume i.e., 70 ml/kg can be chosen and the patient ventilated with at least twice the predicted minute volume i.e. 140 ml/kg. Here a deliberate controlled rebreathing is allowed in order to maintain normal Paco2 along with high humidity, less heat loss and greater economy of fresh gas. Combinations between these two extremes can also be used. It is important to remember that using a low FGF with normal minute ventilation, can lead to hypercarbia; a moderate FGF and hyperventilation, can lead to hypocarbia.
COMBINED SYSTEMS
To over come the difficulties of changing the breathing systems for different modes of ventilation, Humphrey designed a system called Humphrey ADE18, with two reservoirs, one in the afferent limb and the other in the efferent limb. While in use, only one reservoir will be in operation and the system can be changed from ARS to ERS by changing the position of a lever. It can be used for adults as well as children. The functional analysis is the same as Mapleson A in ARS mode and as Bain in ERS mode. It is not yet widely used.
BREATHING SYSTEMS WITH co2 ABSORPTION
Systems so far described have relied on FGF for effective elimination of co2. Any desire to economize on FGF by allowing a total rebreathing, should be accompanied by removal of the expired co2 by chemical absorption using sodalime or baralyme. The systems designed for these purpose are again classified as:
Unidirectional flow.
-Circle system.
Bi-directional flow.
-To and fro system.
The essential components of the circle system are, (1) a sodalime canister, (2) Two unidirectional valves, (3) Fresh gas entry, (4) Y-piece to connect to the patient, (5) Reservoir bag (6) a relief valve and (7) low resistance interconnecting tubing. The arrangement of the components is shown in fig.14. For efficient
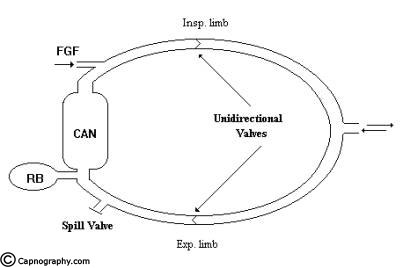
functioning of the system the following criteria should fulfilled. (1) There should be two unidirectional valves on either side of the reservoir bag, (2) Relief valve should be positioned in the expiratory limb only, (3) The FGF should enter the system proximal to the inspiratory unidirectional valve.
Functional analysis: During inspiration the FG along with the co2 free gas in the reservoir bag flow through the inspiratory limb and inspiratory unidirectional valve to the patient. No flow takes place in the expiratory limb as the expiratory unidirectional valve is closed by back pressure transmitted to the valve. During expiration the inspiratory unidirectional valve closes and the expired gas flows through the expiratory unidirectional valve in the expiratory limb to the sodalime canister and to the reservoir bag. The co2 is absorbed in the canister. The FGF from the machine continues to fill the reservoir bag. When the reservoir is full the relief valve opens and the excess gas is vented to atmosphere. By selecting a suitable position for the relief valve, the expired gas can be selectively vented when the FGF is more than the alveolar ventilation. To facilitate controlled ventilation the relief valve has to be partly closed and the excess gas is vented during inspiration. The gas flow pattern is similar to that described above.
The advantages and disadvantages of the various arrangements of the components were analyzed by Eger and Ethans7. The relative positions of the components of the circle system are of particular importance to the functioning of the system only when the FGF is high, the gas components of the system unmixed and co2 absorber not used. When the FGF is reduced below the alveolar ventilation, the co2 absorber is a must as the gas in the system become more uniformly mixed, and the relative position of the system’s components become less important
Totally closed system:
The systems with co2 absorption can be used in a completely closed mode. After a period of approximately 10-20 minutes breathing with high inflow of fresh gas for denitrogenation, the expiratory valve is closed. The FGF is then adjusted to meet only the patients basal oxygen requirements together with anaesthetic. A number of advantages have been demonstrated for totally closed systems.
A) Economy: The FGF could be reduced to as low as 250 – 500 ml of oxygen. The consumption of Halothane/Isoflurane has been found to be around 3.5 ml/hour19.
b) Humidification: In the completely closed system, once the equilibrium has been established, the inspired gas will be fully saturated with water vapour20.
C) Reduction of heat loss: In addition to conserving water the totally closed system will also conserve heat. The co2 absorption is an exothermic reaction and the system may actively assist in maintaining body temperature.
D) Reduction in atmospheric pollution: Once the expiratory valve has been closed, no anaesthetic escapes, except for the small percutaneous loss from the patient.
E) Control of anaesthesia: It is possible to compute the time course of uptake of anaesthetic in a patient of known size and add the appropriate quantity of the anaesthetic to the circuit at a rate decreasing in a manner calculated to maintain a constant alveolar concentration21. In practice an alveolar concentration of about 1.3 x MAC is found to be suitable.
The technique has several potential disadvantages.
i) A greater knowledge of uptake and distribution is required to master closed circuit anaesthesia.
ii) Inability to alter any concentration quickly.
iii) Real danger of hypercapnia may result from, a) an inactive absorber, B) incompetent unidirectional valves and c) incorrect use of absorber bypass.
Bi-directional flow systems
The Waters to and fro system is valveless and conveniently portable. It has been widely used in the past and now is only of historical importance. The reader may refer to any standard text book for furtherdetails.
References:
1. Dorsch, J.A., Dorsch,S.E. Breathing systems II. In understanding anaesthesia equipment. 2nd ed. 1984. Williams & Wilkins.
2. Conway, C.M.: Anaesthetic breathing systems. British Journal of Anaesthesia. 1985, 57, 649-57.
3. Miller, D.M.: Breathing systems for use in anaesthesia. Evaluation using a physical lung model and classification. British Journal of Anaesthesia. 1988, 60, 555-64.
4. Miller DM. An enclosed efferent afferent reservoir system: The Maxima. Anaesthesia and intensive care 1995; 23:284-91.
5. Miller DM. Breathing systems reclassified. Anaesthesia and intensive care 1995; 23: 281-83.
6. Miller DM, Palm A. Comparison in spontaneous ventilation of the Maxima with the Humphrey ADE breathing system and between four methods for detecting rebreathing. Anaesthesia and intensive care 1995; 23: 296-01.
7. Eager EI., Ethans, C.T.: The effects of inflow, overflow and valve placement on economy of circle system. Anesthesiology, 1968, 29, 93-100.
8. Mapleson WW. The elimination of rebreathing in various semiclosed anaesthetic systems. British journal of Anaesthesia; 1954;26: 323-32.
9. White, DC, Calkins, J. Anaesthetic machine and breasting apparatus. In: Nunn, Utting and Brown eds. General anaesthesia. 5th ed.. Butterworths, London 1989:428-56
10. Miller, D.M., Miller, J.C.: Enclosed afferent reservoir breathing systems. Description and clinical evaluation. British Journal of Anaesthesia. 1988, 60, 469-75.
11. Droppert PM, Meakin G, Beatty PCW, Mortimer AJ, Healy TEJ. Efficiency of a new afferent reservoir breathing system during controlled ventilation. British Journal of Anaesthesia 1991; 66: 638-42.
12. Meakin G, Jennings AD, Beatty PCW, Healy TEJ. Fresh gas requirements of an enclosed afferent reservoir breathing system during controlled ventilation in children. British Journal of Anaesthesia 1992; 68: 43-47.
13. Beatty PCW, Meakin G, Healy TEJ. Fractional delivery of fresh gas: a new index of the efficiency of semiclosed breathing systems. British Journal of Anaesthesia 1992; 68: 474-77.
14. Tham EJ, Davies R, Slade JM, Mapleson WW. Efficiency of breathing systems A and D in the Carden Ventmasta ventilator. British Journal of Anaesthesia 1993; 71: 741-46.
15. Ravishankar M, Chatterjee S. Fractional utilisation of fresh gas by breathing systems without carbon dioxide absorption. British Journal of Anaesthesia 1993; 71: 706-07
16. Ward CS. In: Anaesthetic equipment. Physical principles and maintenance; W.B.Saunders, London; 2nd ed. 1985.
17. Rose DK, Froese AB. The regulation of Paco2 during controlled ventilation of children with a T-piece. Canadian Anaesthetists Society Journal 1979; 26: 104-13.
18. Humphrey D. A new anaesthetic breathing system combining Mapleson A,D and E principles. Anaesthesia 1983; 38: 361-72
19. Baum JA, Aitkenhead AR. Low flow anaesthesia. Anaesthesia 1995; 50(supplement):37-44.
20. Kleemann, P.P. Humidity of anaesthetic gases with respect to low flow anaesthesia. Anaesthesia and Intensive Care, 1994, 22 (4), 396-408.

 Twitter
Twitter Youtube
Youtube