Capnography and Laparoscopy
Capnography has three important applications during laparoscopic surgery.
1. It serves as a non-invasive monitor of PaCO2 during CO2 insufflation and therefore can be used to adjust ventilation.
2. It may help in the detection of accidental intravascular CO2 insufflation.
3. It may help in the detection of complications of CO2 insufflation such as pneumothorax and hemorrhage.
Hemodynamic and ventilatory changes during CO2 insufflation
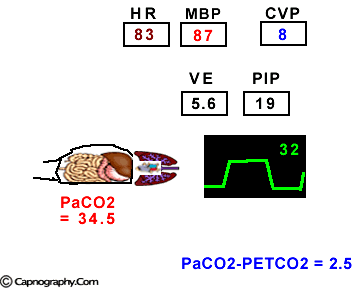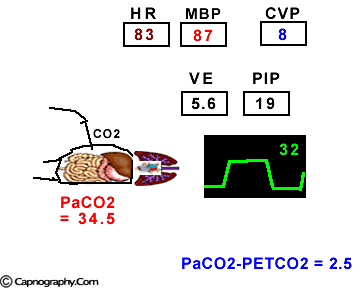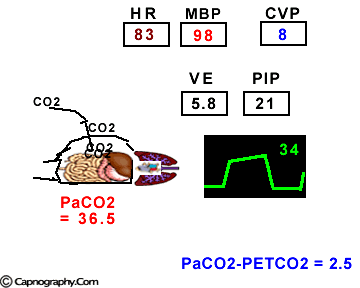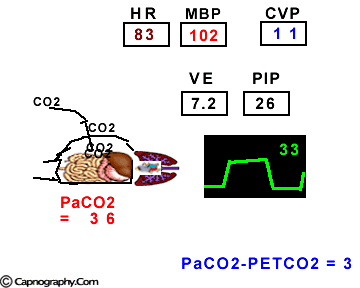
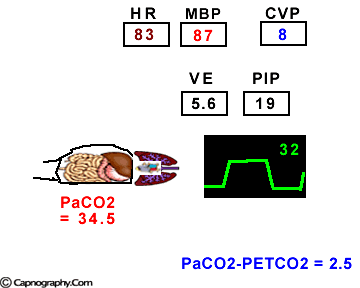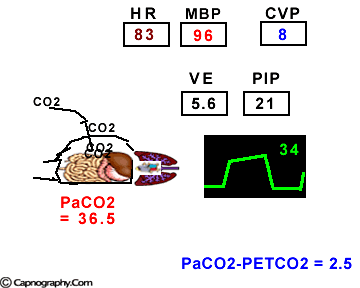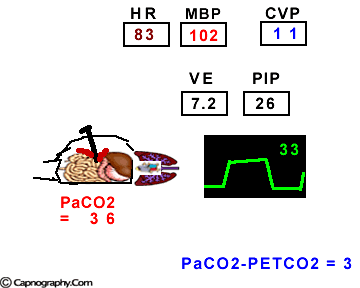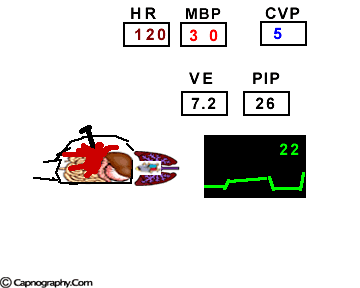 (Based on the references, 1,9,16,17)
(Based on the references, 1,9,16,17)PETCO2 as a non-invasive monitor of PaCO2:
Prolonged intra-abdominal insufflation with CO2 in anesthetized and mechanically ventilated patients during upper abdominal laparoscopic surgery does not significantly affect the reliability of PETCO2 monitoring in predicting PaCO2 in healthy ASA I and II subjects and elderly patients.1-3 However, in ASA III and IV patients, PETCO2 may not reflect changes in PaCO2 during insufflation due to changes in alveolar dead space consequent to reduced cardiac output, increased ventilation-perfusion mismatching, or both.4,5 Therefore, direct arterial PaCO2 monitoring is recommended in patients with significant cardiorespiratory diseases. An arterial line is reasonable to monitor PaCO2 in ASA III and IV patients for three reasons: (1) end-tidal PCO2 is not a reliable index of PaCO2, (2) the normal gradient of 3 to 5 mm Hg between PaCO2 and PETCO2 is increased, and (3) even with normal PETCO2, achieved by increasing minute volume, PaCO2 may be as high as 50 mm Hg resulting in respiratory acidosis.
Pregnant patients:
The Society of American Gastrointestinal Endoscopic surgeons (SAGES) published guidelines for laparoscopic surgery during pregnancy that include perioperative monitoring of arterial blood gases as well as perioperative fetal and uterine monitoring.6 This belief has been echoed by other authorities.7-9 Amos etal,7 documented four fetal deaths in seven pregnant women who underwent laparoscopic cholecystectomy or appendectomy (in these patients the ventilation was adjusted to maintain PETCO2 in low-to mid-30’s). Although Amos et al, did not perform arterial blood gases, respiratory acidosis was stated as a possible factor contributing to fetal loss.10 Based on studies in pregnant ewes, 12,13 these concerns stem from previous studies indicating that elevation in maternal PaCO2 could impair fetal CO2 excretion across the placenta and could exacerbate fetal acidosis. Other risk factors were present for fetal loss in this series, including perforated appendix and pancreatitis.7
The pregnant sheep model gives further credence to these concerns. Hunter et al9 and Cruz et al8 both observed that CO2 pneumoperitoneum resulted in a PaCO2-PETCO2 gradient of 16 to 25 mm Hg. The resulting fetal hypercarbia correspondeds to to fetal hypertension, acidosis, and tachycardia. Hunter’s group also demonstrated that the PETCO2 lagged behind the PaCO2 by approximately 60 minutes, and hyperventilation was not able to prevent hypercarbia and acidosis. Because of the large changes in PaCO2 levels, these two groups reported that capnography might underestimate and is therefore not a reliable indicator of respiratory acid-base status during CO2 insufflation. They recommended arterial blood gas monitoring for all parturients undergoing laparoscopic surgery; however, Bhavani-Shankar and Mushlin10 challenged the appropriateness of the ewe model for this purpose. Subsequently, Bhavani-Shankar et al11 prospectively evaluated the PaCO2-PETCO2 difference in eight parturients undergoing laparoscopic cholecystectomy with CO2 pneumoperitoneum. The intra-abdominal pressures were maintained around 15 mm Hg. These women underwent surgery with general anesthesia during the second and third trimester of their pregnancies. Adjusting minute ventilation to maintain PETCO2 at 32 mm Hg, the arterial blood gases were measured at fixed surgical phases: preinsufflation, during insufflation, postinsufflation, and after completion of anesthesia. The authors found no significant difference in either mean PaCO2-PETCO2 gradient or PaCO2 and pH during the various phases of laparoscopy as shown below.
| Pre-insufflation | During insufflation | Post-insufflation | Post-extubation | Post-Anesth. Care unit |
|
| PETCO2
(mmHg) |
32.1 (1.6)
N =17 [31.3-32.8] |
32.4 (1.1)
N =28 [31.9-32.8] |
32.7(1.4)
N =12 [31.9-33.5] |
||
| PaCO2
(mmHg)
|
34.5 (2.6)
N =17 [33.2-35.7] |
35 (1.7)
N =28 [34.3-35.6] |
34.6 (2.5)
N =12 [33.1-36] |
37.9 (2.8)
N =4 [35.1-40.6] |
36.2 (2.9)
N =8 [34.1-38.2] |
| PaCO2-PETCO2
(mmHg) |
2.4 (1.5)
N =17 [1.6-3.1] |
2.6 (1.2)
N =28 [2.1-3] |
1.9 (1.4)
N =12 [1.1-2.6] |
This is in contrast to results obtained by Cruz et al (9 pregnant ewes) and Hunter et al (4 pregnant ewes) where the difference increased by a mean of 10 mmHg during insufflation.12,13 Among the twenty-eight observations obtained in 8 patients during CO2 insufflation in our study, the highest PaCO2-PETCO2 observed was 5.1 mmHg as against 16 and 25 mmHg observed by Cruez et al and Hunter et al in ewes.8.9 The results observed in our study are consistent with our earlier observations of derived PaCO2-PETCO2 of 6-7 mmHg from tcPCO2 monitoring in laparoscopic surgery in a parturient.12 Therefore, the physiologic consequences of pneumoperitoneum are different in humans from those in pregnant ewes as seen by a lowered PaCO2-PETCO2 during insufflation in pregnant patients.
Although the sheep model is used for obstetric research, there could be physiological differences between the two species. For example, the preinsufflation PaCO2-PETCO2 in pregnant ewes range from 6 to 15 mmHg,8,9 whereas they are lower in pregnant humans (0.6 mmHg, range -2.5-5.1 mmHg; occasionally PETCO2 exceeds PaCO2).13-15 In our study, they varied from 0 to 5 mmHg. These values are similar to the values reported by the author in pregnant subjects undergoing cesarean section and in women during postpartum sterilization.13,14 The smaller pre-insufflation arterial to end-tidal PCO2 difference in humans translates into a lower preinflation alveolar deadspace in pregnant patients than in pregnant ewes.15 A lower preinsufflation alveolar deadspace in pregnant patients probably results in a smaller subsequent changes in alveolar deadspace during CO2 insufflation, and thus to a smaller PaCO2-PETCO2 in pregnant patients than in pregnant ewes.
Hence it appears that arterial blood gas monitoring during laparoscopy in pregnant patients with healthy lungs may not be necessary.
Infants and children
Rosinoso-Barbero et al concluded that capnography proved to be an excellent guide to adjust ventilation during CO2 insufflation in infants and children.18 However, Laffon et al concluded that PETCO2 monitoring may overestimate PaCO2 and consequently can result in hyperventilation during laparoscopic surgery.19 Arterial to end-tidal carbon dioxide differences were studied in sixty-one children undergoing pneumoperitoneum under general anesthesia.19 At a steady state, before pneumoperitoneum, the mean (a-ET)PCO2 was -1.2 (SD)2.2, (confidence interval -5.6 to +3.4) Hg. At a steady state during CO2 insufflation, the mean difference was -2.0(SD),(confidence interval -8.8 to +4.8) mm Hg although PaCO2 and PETCO2 increased by about 14% from baseline values. The incidence of negative gradients increased from 54% at pre-insufflation to 67% during CO2 insufflation. The authors concluded that PETCO2 often overestimates PaCO2 during laparoscopy in children, by up to 8.8 mm Hg and therefore concluded that arterial blood gas analysis should be performed during long procedures to avoid hyperventilation. A limited data presented in a study by Bozkurt et al shows no changes in (a-ET)PCO2 at 30 during CO2 insufflation compared to baseline levels.20
Negative (a-ET)PCO2:
Occasionally, during the course of laparoscopic surgery, the gradient may be negative in adults, in which case PETCO2 overestimates PaCO2.1 The incidence of negative gradients is higher in infants and children undergoing laparoscopic surgery.19 Low frequency, high tidal volume ventilation will open hitherto closed alveoli, whose CO2 will now be seen as an increase in the slope of phase III of the capnogram and PETCO2 will be close or exceed the PaCO2 line. Low FRC coupled with increased CO2 delivery to the alveoli can exacerbate this response thereby increasing the frequency of occurrences of negative (a-ET)PCO2 values during laparoscopic surgery.13-15
References:
1. Wabha RWM, Mamazza J. Ventilatory requirements during laparoscopic cholecystectomy. Can J Anaesth 1993;40:206-210.
2. Nyarwaya JB, Mazoit JX, Samii K. Are pulse oximetry and end-tidal carbon dioxide tension monitoring reliable during laparoscopic surgery? Anaesthesia 1994;49:775-778.
3. Baraka A, Jabbour S, Hammoud R et al. Can pulse oximetry and end-tidal capnography reflect arterial oxygenation and carbon dioxide elimination during laparoscopic cholecystectomy? Surg Laparosc Endosc 1994;4:353-356.
4. Feig BW, Berger DH,Dougherty TB et al. Pulmonary effects of CO2 abdominal insufflation (CAI) during laparoscopy in high-risk patients. Anesth Analg 1994;78(supplement):S108.
5. Monk TG, Weldon BC, Lemon D. Alterations in pulmonary function during laparoscopic surgery. Anesth Analg 1993;76(Supplement):S274.
6. Guidelines for laparoscopic surgery during pregnancy. Surg Endosc 1998;12:189.
7. Amos JD, Schorr SJ, Norman PF, et al. Laparoscopic surgery during pregnancy. Am J Surg 1996;171:435.
8. Cruez AM, Southerland LC, Duke T, et al. Intra-abdominal carbon dioxide insufflation in the pregnant ewe. Anesthesiology 1996;64:790.
9. Hunter JG, Swanstrom L, Thronburg K. Carbon dioxide pneumoperitoneum induces fetal acidosis in pregnant ewe model. Surg Endosc 1995;9:272.
10. Bhavani-Shankar K, Mushlin P. Arterial to end-tidal gradients in pregnant subjects. Anesthesiology 1997;87:1596.
11. Bhavani shankar K, Steinbrook R, Brooks D, Datta S. Arterial to end-tidal carbon dioxide difference during anesthesia for laparoscopic surgery in pregnancy. Anesthesiolgy 2000;93(2):370-3.
12. Bhavani Shankar K, Steinbrook RA, Mushlin PS, Freiberger D. Transcutaneous PCO2 monitoring during laparoscopic cholecystectomy in pregnancy. Canadian J Anaesth 1998;45(2):164-9.
13. Bhavani Shankar K, Moseley H, Kumar Y, Vemula V, Krishnan A. Arterial to end-tidal carbon dioxide tension difference during anaesthesia for tubal ligation. Anaesthesia 1987;42:482-6.
14. Shankar KB, Moseley H, Kumar Y, Vemula V. Arterial to end-tidal carbon dioxide tension difference during Caesarean section anaesthesia. Anaesthesia 1986; 41:698-702.
15. Bhavani Shankar K, Moseley H, Kumar Y, Delph Y. Capnometry and anaesthesia. Can J Anaesth 1992;39:6:617-32.
16 Wahba RW, Beique F, Kleiman SJ. Cardiopulmonary function and laparoscopic cholecystectomy. Review article. Can J Anaesth 1995;42:1:51-63.
17 Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg 1993;76:1067-71,
18 Resinoso-Barbero F, Diez A Paz JA, et al. Physiopathologic implications of the anesthesiologic management of pediatric laparoscopic surgery. Rev Esp Anesthesiol Reanim 1995;42:277-82.
19. Laffon M, Goucher A, Sitbon P et al. Differences between arterial to end-tidal carbon dioxide pressures during laparoscopy in paediatric patients. Can J Anaesth 1998;45:561-3.
20. Bozkurt P, Kaya G et al. The cardiorespiratory effects of laparoscopic procedures in infants. Anaesthesia 1999;54:831-4.

 Twitter
Twitter Youtube
Youtube









