Capnography tips
Discipline is the key to success. In anesthesia practice, this translates to a global assessment of a problem in resolving an anesthesia crisis. Let capnography play its role in helping you to reach a diagnosis as quickly as possible. You can derive maximum benefit from the science of capnography if you follow some of these guidelines in day to day anesthesia practice.
Calibration of the monitor
Maintain and calibrate the monitor as per manufacturer’s guidelines. For accurate measurements capnographs should be calibrated first, zeroing the monitor to room air, and then administering a gas of known CO2 concentration. As the changes in barometric pressure affect the PETCO2 measurements, calibration procedures should be performed using the same type of sampling tube as will be used when the analyzer is connected to the patient sampling. Omission of standard narrow 2 m long sampling tube (at the time of calibration) will result in the absence of a large pressure drop across the ends of the tube, and a measuring error does becomes inevitable during subsequent clinical use.
Checking the monitor
| Testing a capnometer |
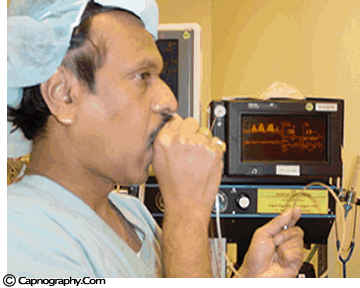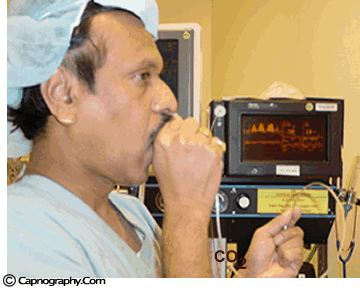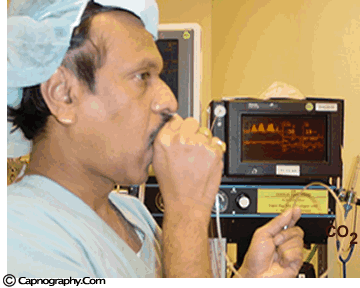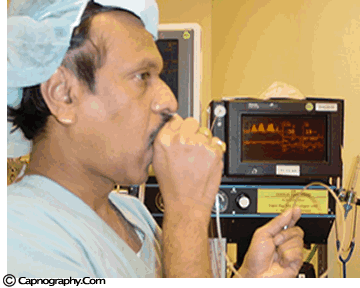 |
Before any interpretations are made of PETCO2 readings and waveforms one should ascertain that the capnograph is functioning correctly. Contamination of the unit with secretions or mucus may cause distortion of CO2 waveforms. Similarly partial obstruction of sampling lines with water can result in distorted waveforms. A leak in the sampling tube may result in low PETCO2 measurements. Check the monitor to see if is functioning properly. A simple, but less accurate, method is to record a normal CO2 tracing (eg., one’s own). The typical CO2 waveform with PETCO2 readings between 38-42 mm Hg, confirms the proper functioning of the capnograph.
Position the monitor higher than the patient
| Low level of the monitor may facilitate condensed water and secretions move towards the analyzer thereby resulting in blockade of filters | Monitor above the level of the patient may facilitate condensed water and secretions not go up the sampling tube |
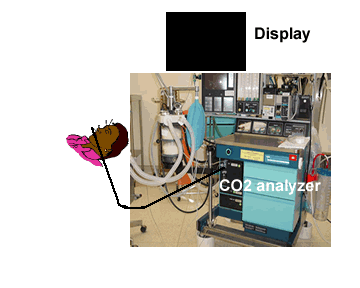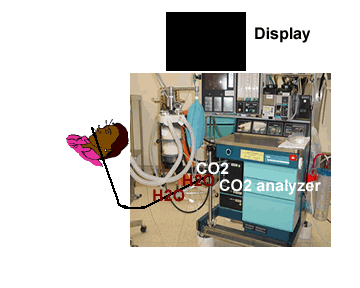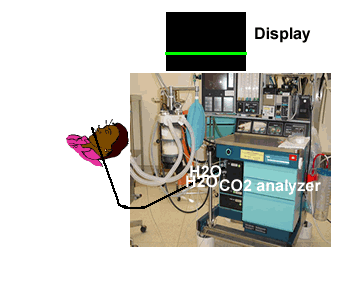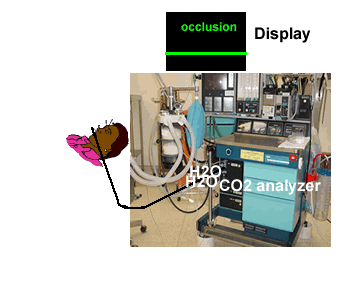 |
 |
It is preferable to keep the capnograph above the level of the patient. This would prevent secretions and water dribbling down the tube towards the monitor end and block the filters frequently.
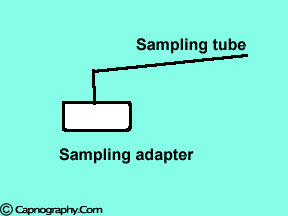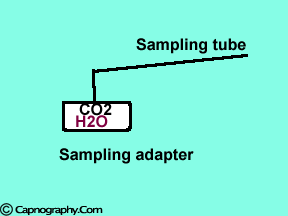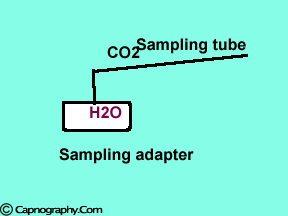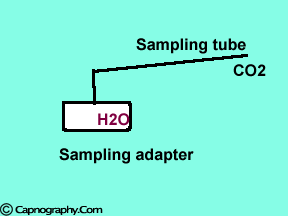
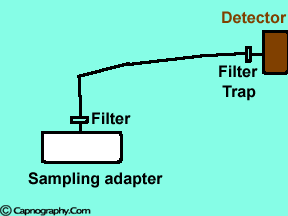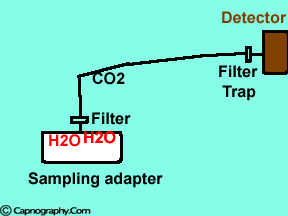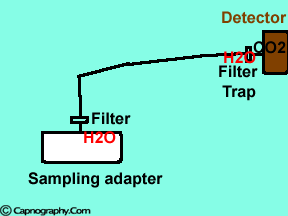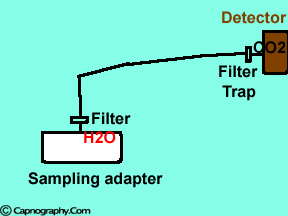
Position the sampling tube upwards and use filters
| Position the sampling vertically upwards | Use water filters at both ends of sampling tube |
 |
 |
Condensation of water, secretions, therapeutic aerosols and water aerosols form humidifier
Water droplets, patient secretions and coalescence of water aerosols from humidifiers in breathing circuits may result in an accumulation of water and secretions in the breathing hoses. The contaminants enters the sampling tubes and increases flow resistance in the tubing thus affecting accuracy of the CO2 measurements. The sampling tubes may also be occluded. Some units either increase the sampling flow or reverse the flow (purge) when a drop in pressure from a flow restriction is sensed. This will help to clear the secretions from the tube. If the occlusion is not cleared the sampling tube must be replaced. Some times, liquids can enter the main unit of the analyzer despite the presence of water traps where they can cause corrosion and form residues. This can degrade the performance of the CO2 analyzer. Positioning the sampling tube upwards away from the patient decreases the frequency with which liquids are drawn into the tubes. Interposing filters at either ends of the sampling tube can also minimize the contamination of the CO2 monitor.
New Capnography Monitors:
In the earlier versions of capnographs, filters are necessary to decreases the chances of moisture contaminating the device. The newer capnographs have better moisture trapping mechanisms to prevent capnograph contamination. Furthermore, interposing filters as above may cause distortion of capnograms, particularly the baseline. The capnogram mimics as if there is rebreathing. Therefore, the user is required to use their judgment and experience.
Our Department Recommendations
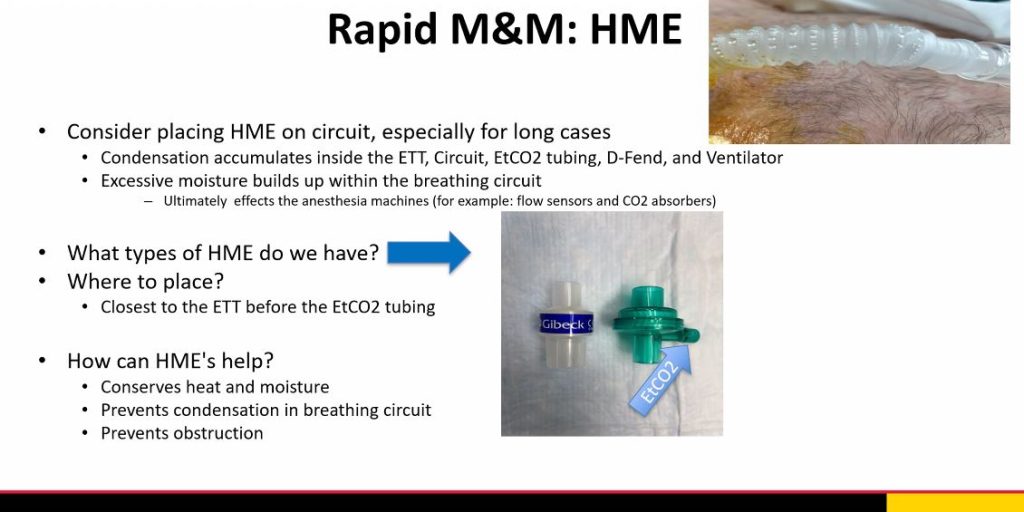
References:
Bhavani Shankar et al. Capnometry and anaesthesia. Canadian J Anaesth 1992;39:617-32.
Adam AP. Capnography and pulse oximetry. In: Atkins RS, Adams AP (Eds.). Recent advances in Anaesthesia and Analgesia. London:Churchill Livingstone, 1989:155-75.
Morgue LR, Rantala B. Capnometers. J Clin Monit 1988;4:115-21.
Carbon dioxide monitors. Health Devices 1986;15:355-85.
Bhavani Shankar K. A method to prevent occlusion of CO2 sampling tubes. Can J Anaesth 1997:44.

 Twitter
Twitter Youtube
Youtube









